STEPS
TOOLS

Measure 6 grams of colloidal TiO2 powder.

Add 9 mL vinegar in 1 mL increments. Grind it in a mortar and pestle.

Alternately, it can be mixed with a spatula in a small beaker.

The mixture should be uniform and lump free, with the consistency of a thick paint.

To the TiO2 paste, add a drop of dish detergent. Do not grind or agitate after the detergent is added.

Let the mixture sit for at least fifteen minutes to equilibrate.

Clean two glass conductive plates by rinsing them in ethanol and then drying them with a soft tissue.

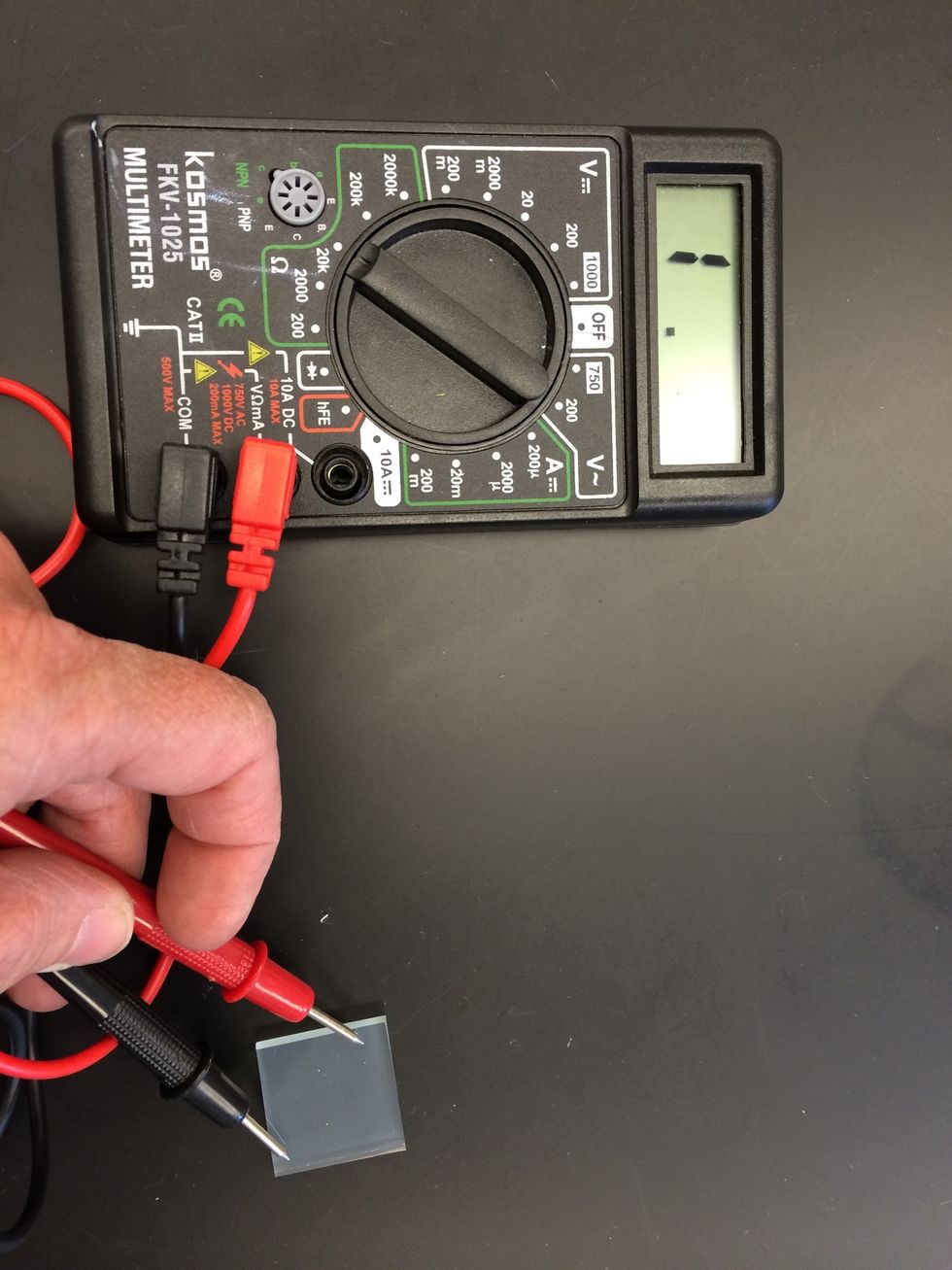
Determine which side of glass is conductive using a multimeter set to ohms.

Orient the glass plate with the conductive side up. Place adhesive tape on three sides of the glass plate to mask a one mm strip on the edges.

Using a pipette, apply a thin line of the TiO2 suspension to the edge of the plate.

Hold a glass stirring rod horizontally by the edge with the strip of TiO2.

Slide the glass stirring rod over the plate to spread and distribute the material. Complete this step within a few seconds after application, before the TiO2 dries.

If the coating does not look uniform, the material can be wiped off the plate with a damp tissue. The deposition should bd repeated with a clean glass rod.

Anneal the TiO2 on the conductive glass using a gas burner. Place the film on a ring stand at the tip of the flame for 10-15 minutes.

During heating, the film may turn brown, then back to white again.

Alternately, the TiO2 coated plates can be heated on a hot plate for 10-15 minutes.

When the annealing is complete, allow the TiO2 coated conductive glass to cool to room temperature. Allow approximately 15 minutes (or the glass may crack and the film may peel).

Prepare the tea or fruits to be used as dyes.

Make strong solutions of each tea.
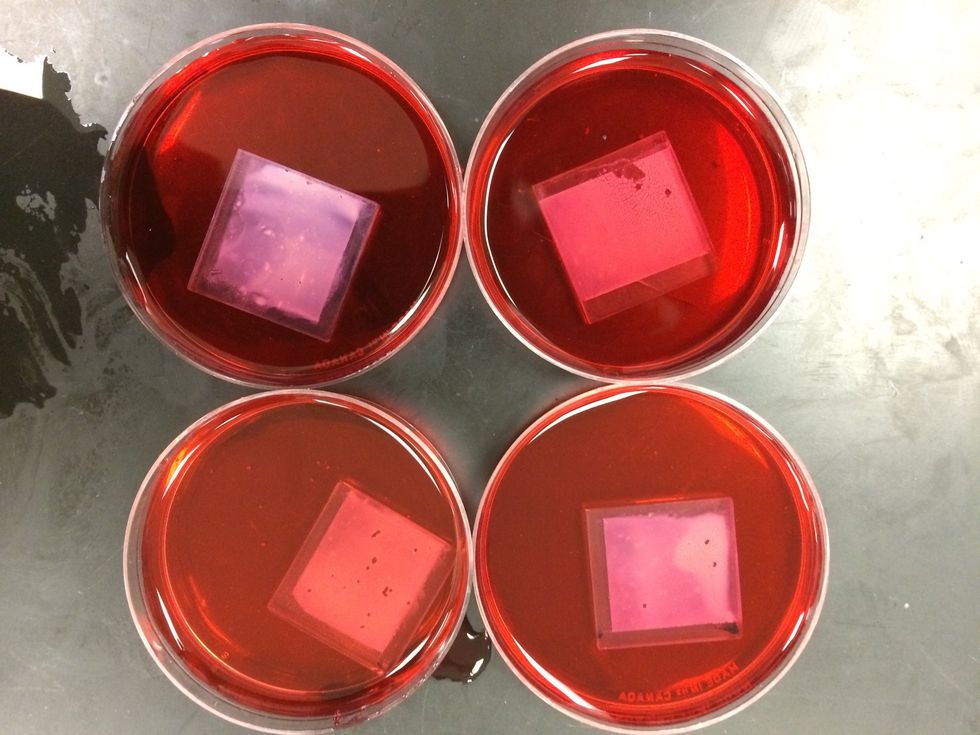
Pour the solution in a Petri dish. Place the TiO2 coated conductive glass face down in the solution for 15 minutes.
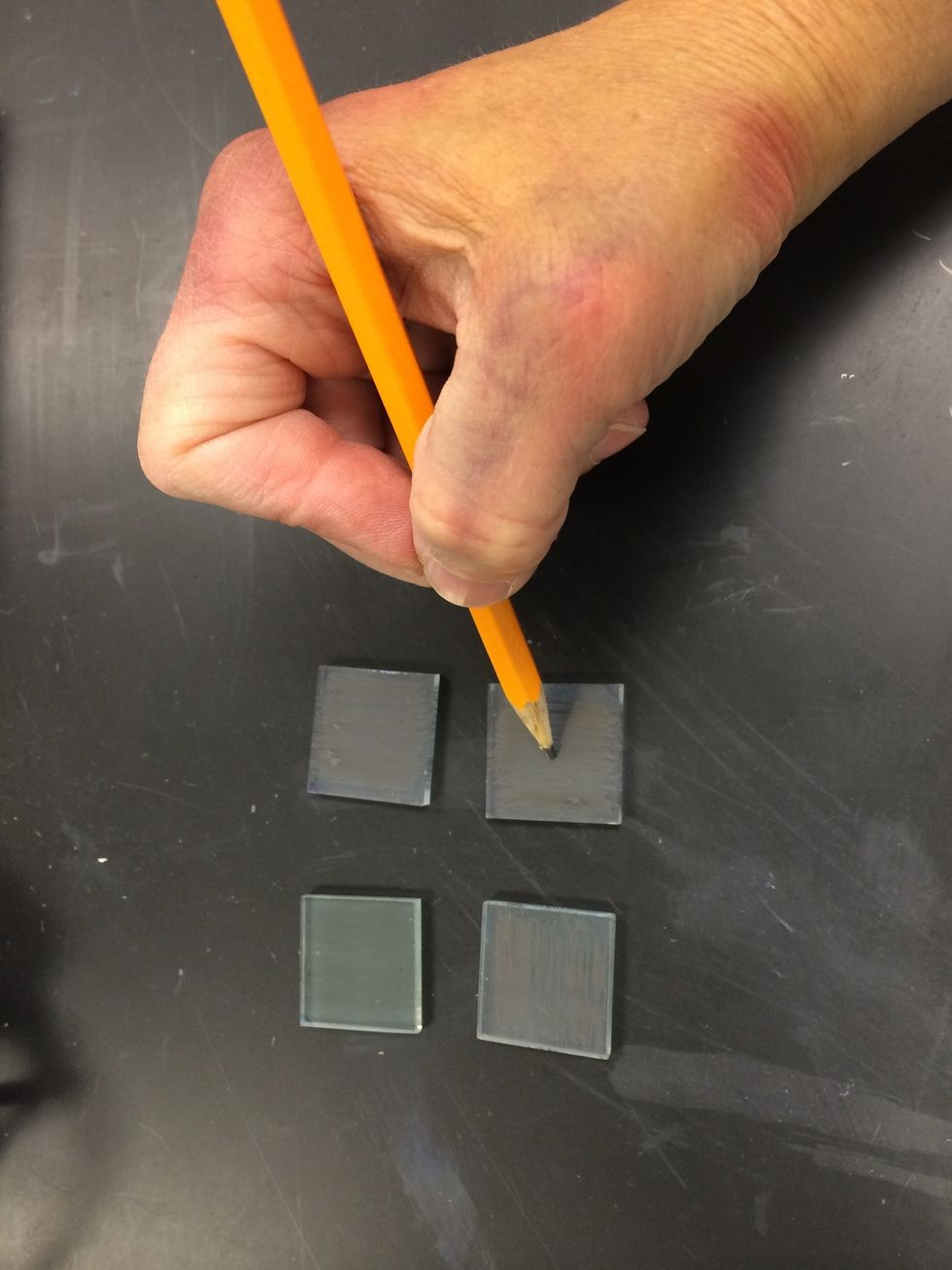
While the TiO2 electrode is being stained, make the counter electrode from the other piece of glass. Using a soft pencil or graphite rod, apply a light carbon film to the entire conductive side.

Be careful not to miss any spots.

Alternately, or in addition, the conductive surface can be coated in soot by holding the plate conductive side down over a candle flame using tweezers.
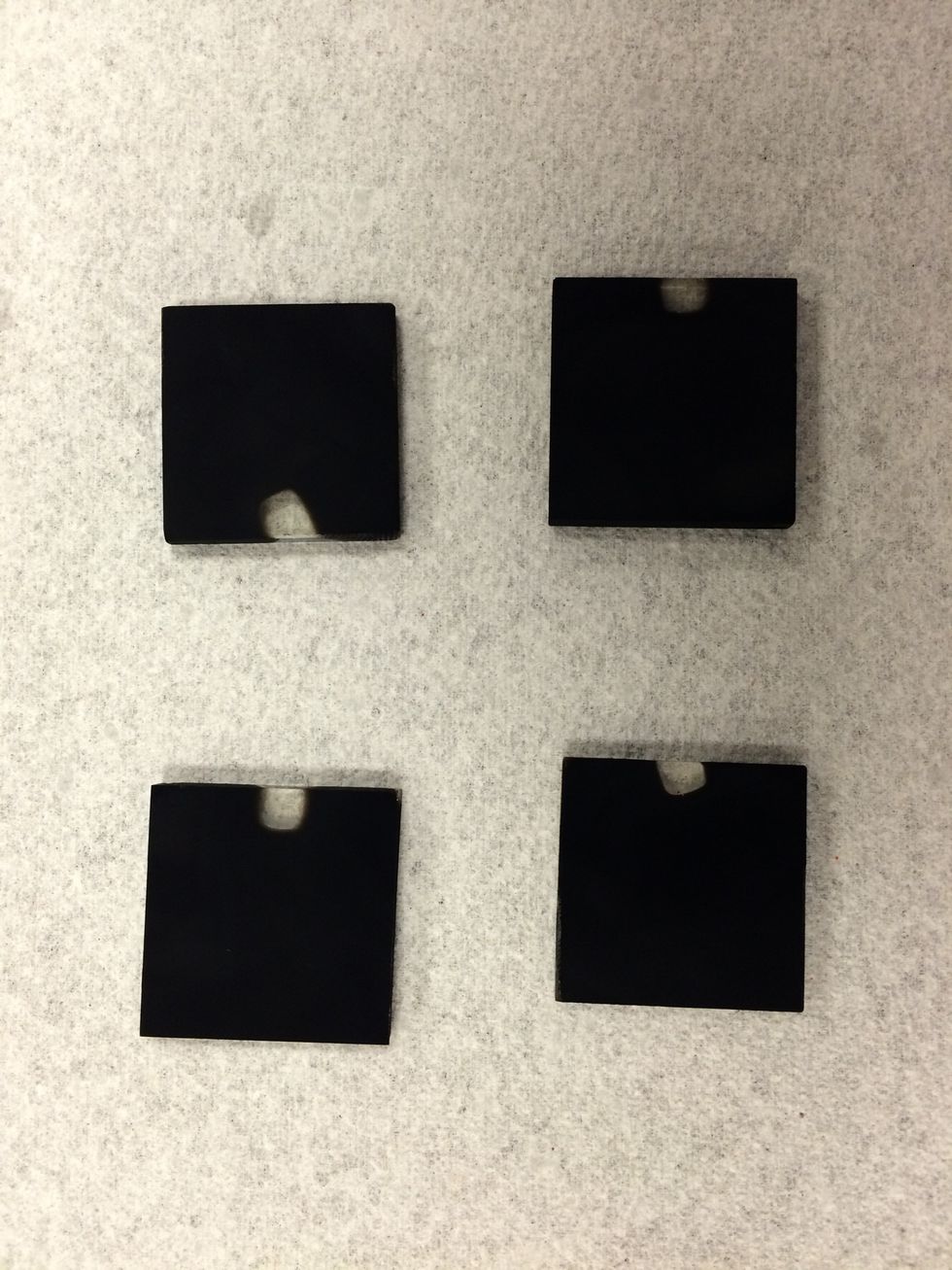
Apply a light even coat over the conductive side of the glass.
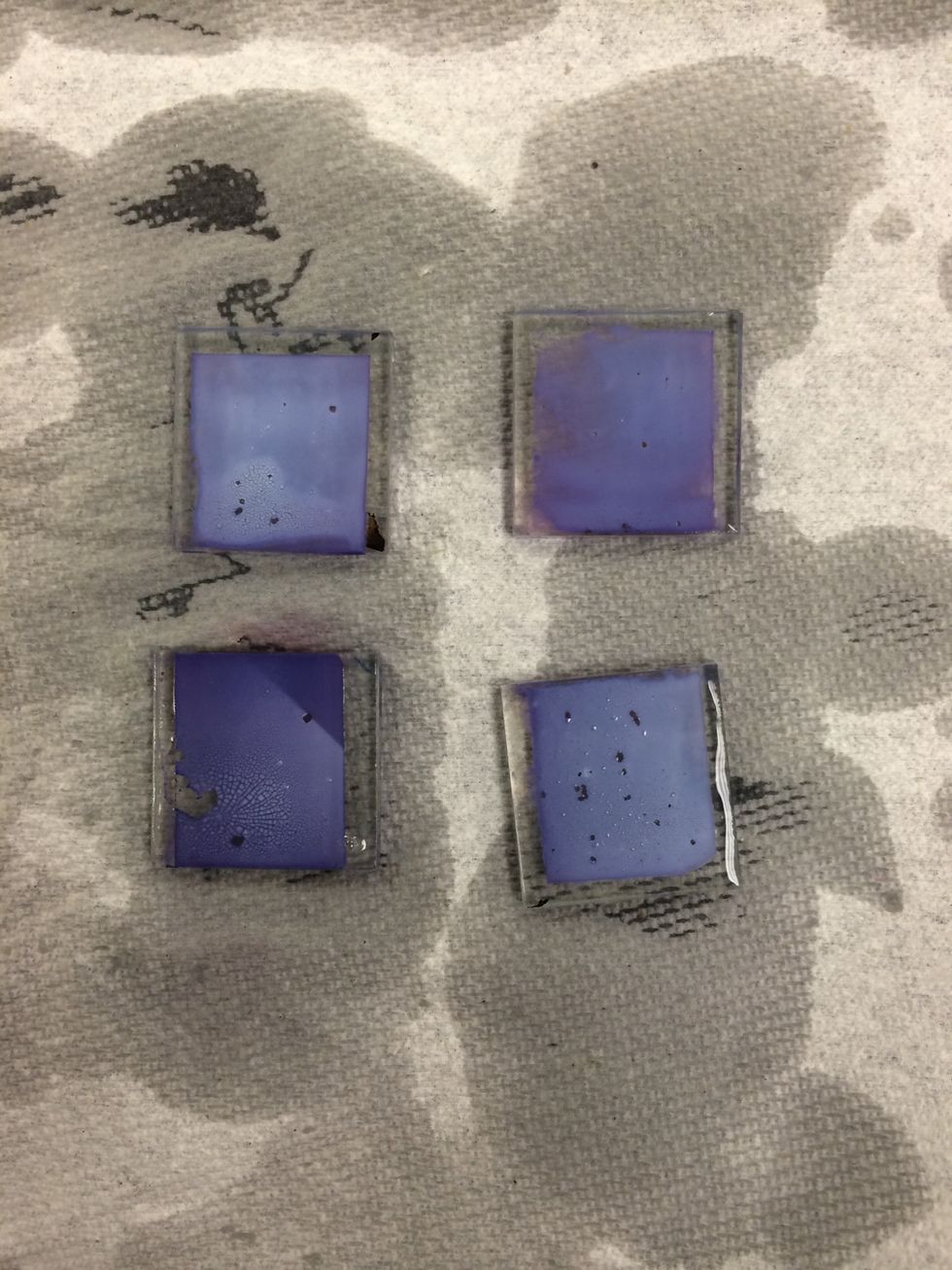
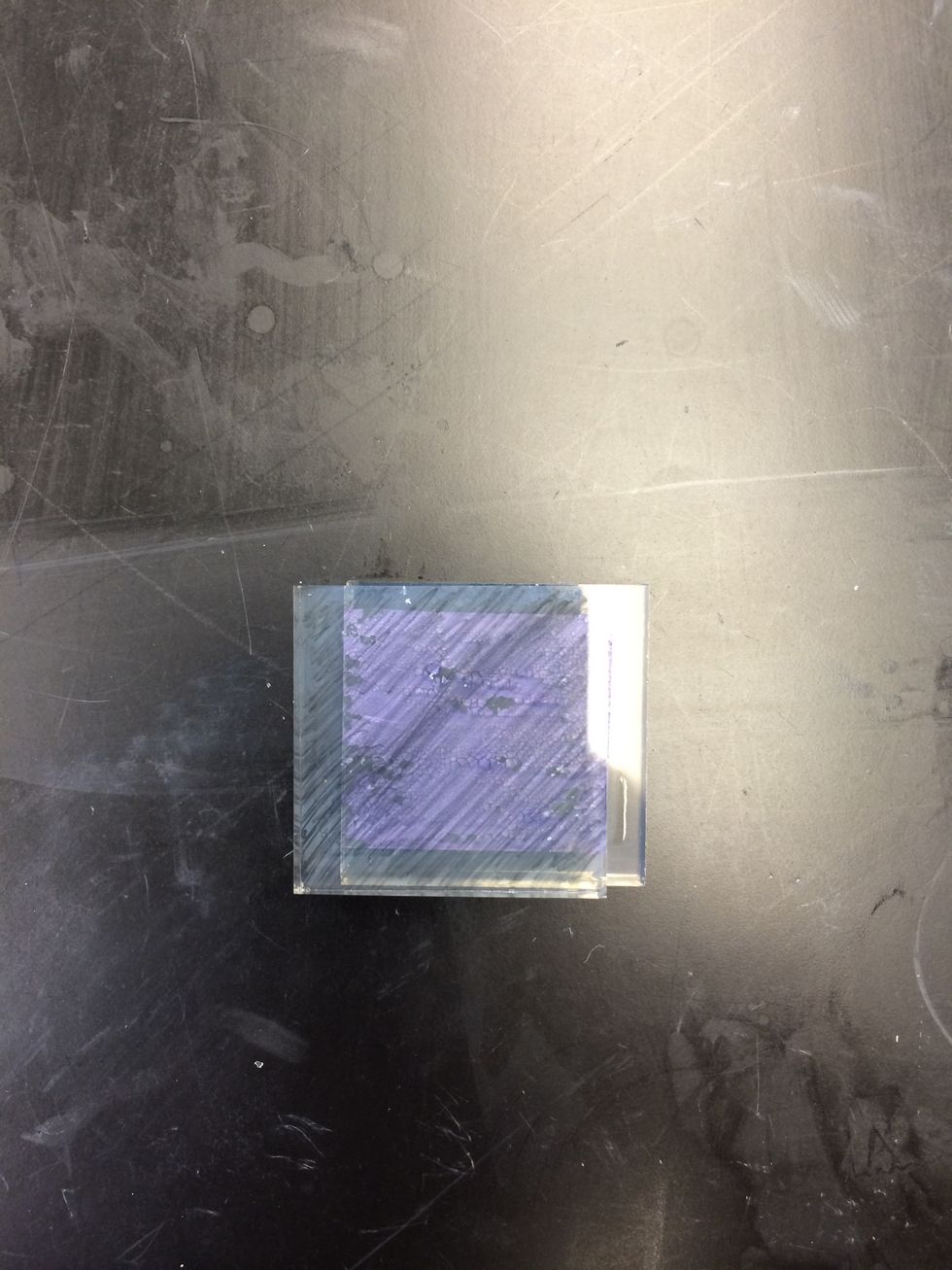
When the TiO2 coated glass has finished soaking, it should be stained dark purple.

Rinse the slides in water, then in ethanol or isopropyl alcohol.

Placed the dried electrode on a flat surface with the dyed TiO2 film side faceup.
Place the carbon coated counter-electrode on top of the TiO2 film plate so that the conductive sides are facing each other.
Shift the plates so that the uncoated edge of the TiO2 plate is exposed. Place two binder clips on the edges to hold the plates together.

Carefully add one or two drops of the iodide electrolyte solution to the edge of the plate.

Alternately open and close each side of the solar cell by releasing and returning the binder clips. This will allow the liquid to be drawn into the space between the electrodes. Wipe of any excess.
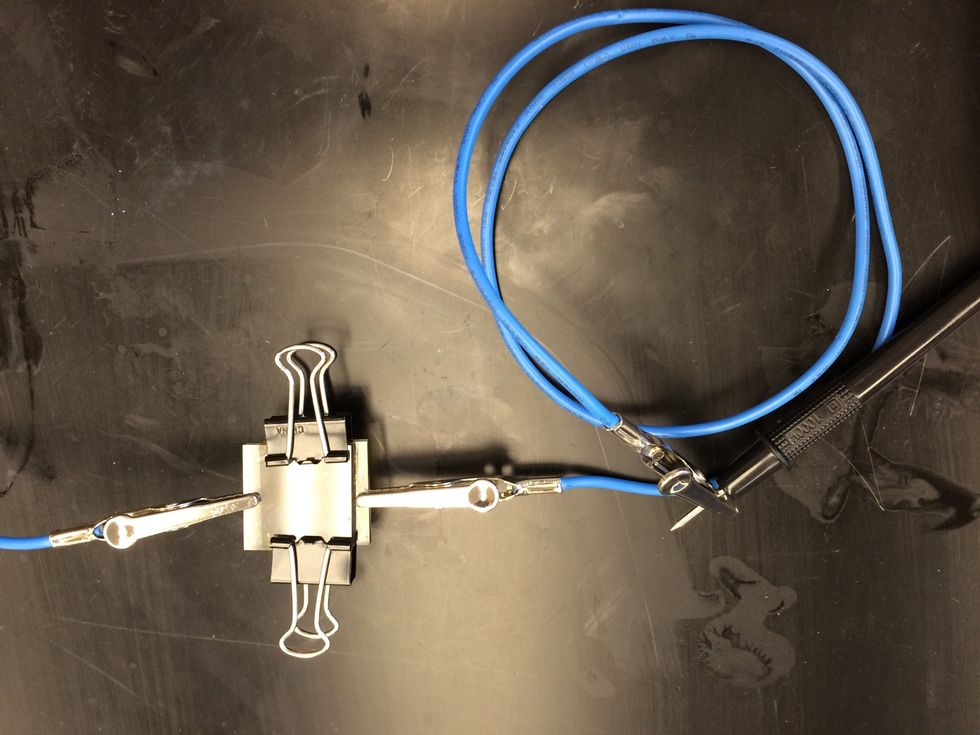
Use the multi meter to test your Gratzel cell. Attached the negative terminal to the edge of the TiO2 coated slide.

Attach the positive lead to the edge of the carbon coated slide.
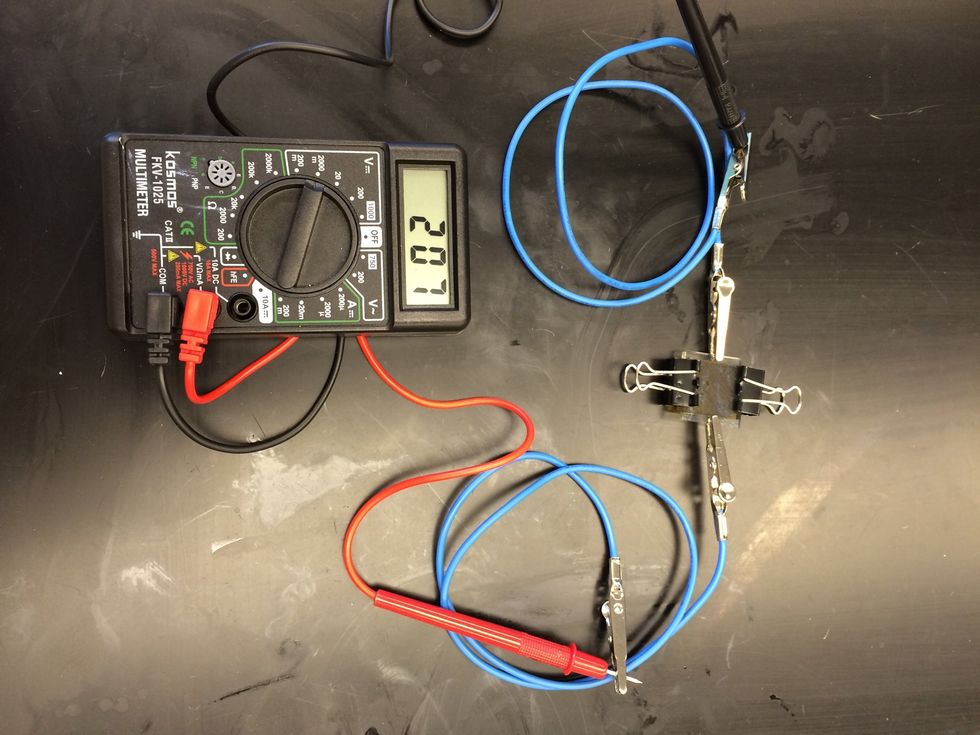
Use the multimeter to record the voltage in various settings.



- Titanium dioxide
- Vinegar
- Conductive transparent glass plates
- Dishwashing detergent
- Dyes: tea, berries , etc
- Iodide electrolyte solution
- Binder clips
The Conversation (0)
Sign Up