The Conversation (0)
Sign Up

I'm hoping this guide will help you depict characteristics of elements based off seeing the periodic table. 😊
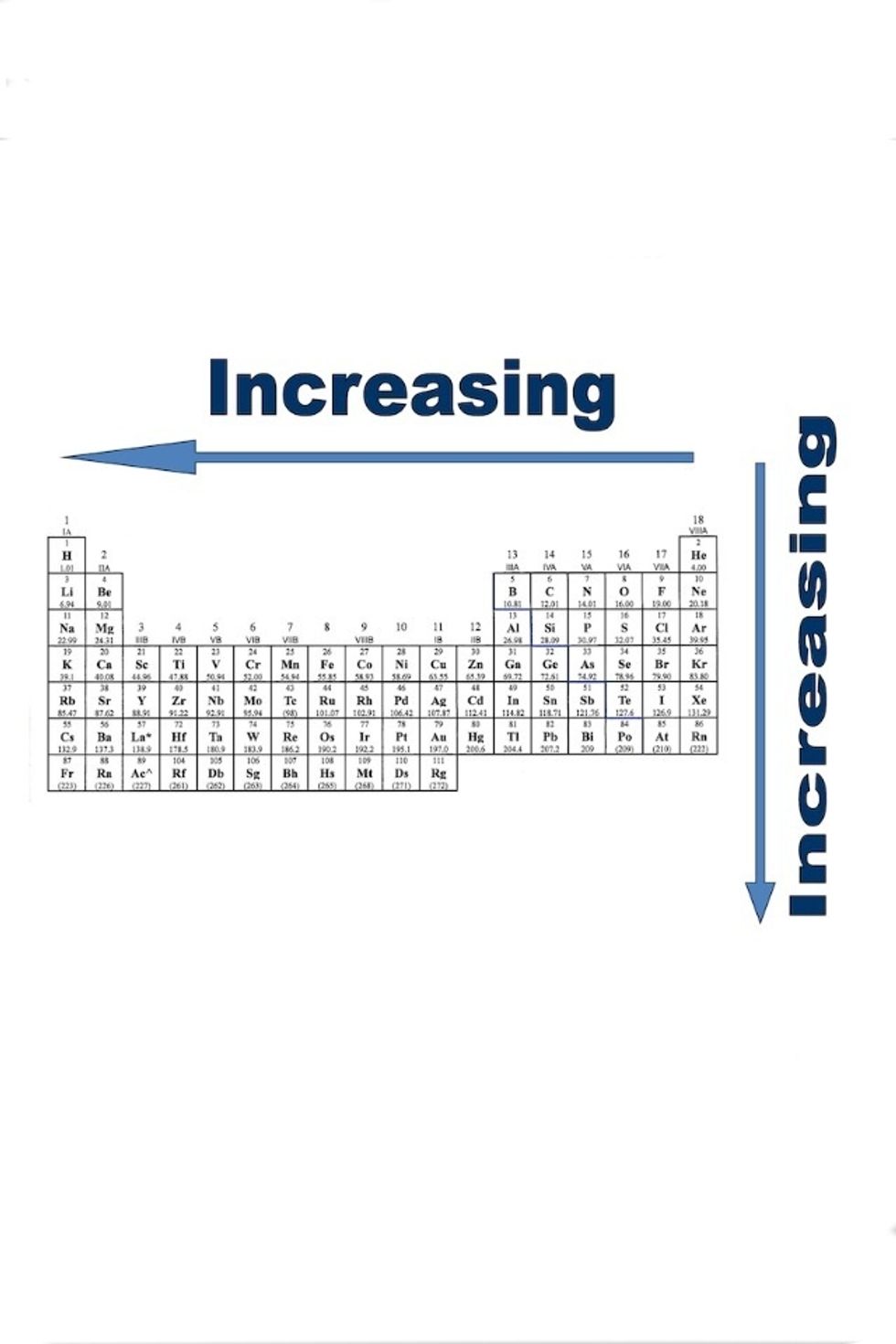
Atomic radius: the distance from the nucleus of the atom to the outermost, stable electron orbital to maintain equilibrium.

Electronegativity: the measurement of the ability an atom has to attract electrons.

Ionization Energy: the amount of energy is needed to remove an electron from an atom.
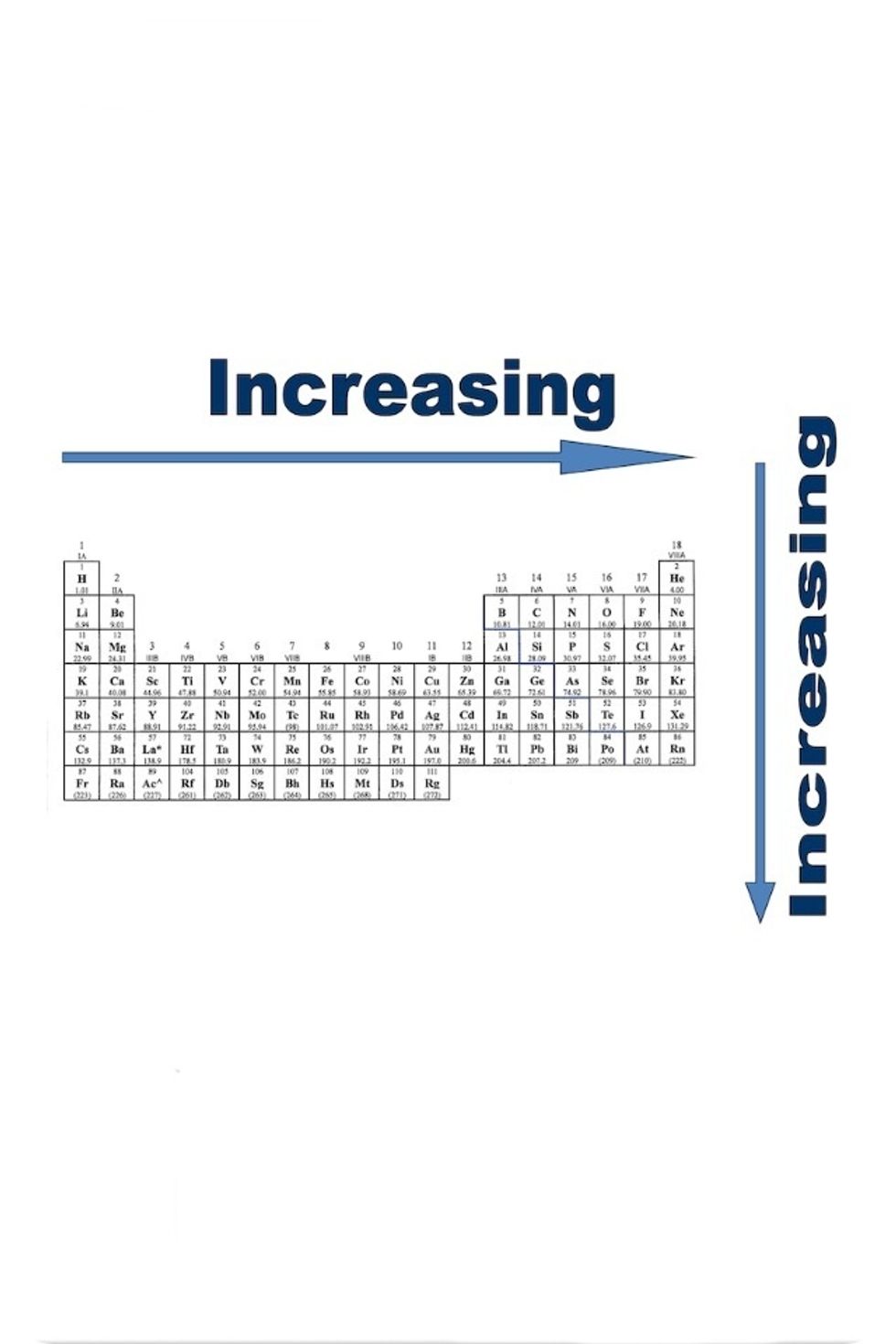
Nucleophilicity: the ability for an atom to donate its electrons.
I hope this guide helped a little on the basic trends of the periodic table! 😃 If there are any comments or questions, please leave them!